WHAT YOU WILL LEARN IN THIS ARTICLE:
- What Is In Vitro Pharmacology?
- What Is Its Role in the Drug Discovery & Development Cycle?
- In Vitro Pharmacology in Drug Discovery
- In Vitro Pharmacology in Pre-clinical Trials
- Can We Fully Replace Animal Testing?
The commercialization of a new drug or medicine takes an average of 10 to 15 years. It is indeed an incredibly costly and complex process that involves multiple rounds of assays and tests. This is because bringing a new drug to market is extremely regulated by dedicated agencies (the EMA in the European Union and the FDA in the United States, just to mention some examples).
The considerations of efficacy and safety must be taken into account throughout the entire drug development process, from the screening of candidate molecules and the identification of the lead compound until the very last stage of pre-clinical trials. Thus, to generate high-quality data on the safety and efficacy of their drug candidates, researchers very often take advantage of in vitro pharmacology.
In this article, you will learn about the role of in vitro pharmacology studies at the different stages of drug discovery and development.
What Is In Vitro Pharmacology?
To understand the concept of in vitro pharmacology it is usually useful to split it in two:
-
in vitro, which refers to studies or experiments conducted on microorganisms and cells outside of their normal biological environment
-
pharmacology, which is the study of the effects of drugs and pharmaceuticals on living organisms
Putting them together, it is now easy to see that in vitro pharmacology is nothing other than the research of the biological effects of drugs and pharmaceuticals, conducted outside of living organisms (in opposition to research performed on living animals or humans).
But what is the purpose of these studies? What information do they generate? How do they help the pharmaceutical field? To answer these questions, we first need to get familiar with the drug discovery and development process, i.e., to understand how drugs are created, to see next where the in vitro pharmacology fits in that process.
What Is Its Role in the Drug Discovery and Development Cycles?
Any new drug or pharmaceutical that enters the market is the result of long and rigorous development, testing, and approval processes.
After the COVID-19 Pandemic, many people became familiar with the different phases of clinical trials, in which new drugs are tested in humans to collect data on drug safety and efficacy. However, the long and bumpy road that precedes it, which takes from the identification of a drug candidate to its production for clinical evaluation, is usually less known.
In Vitro Pharmacology in Drug Discovery
Before drug development comes drug discovery, the process by which new candidate drugs are identified.
Target identification
Fundamental science helps researchers understand how a disease or condition starts and progresses, thus making it possible to conceive new ways of interfering with these pathological processes. This is done by identifying therapeutical targets (a cellular structure, a surface receptor, or a transcription factor, just to name a few examples) against which the drug candidates are expected to act, both selectively and effectively.
Hit discovery
Target identification is then followed by the screening of large libraries of compounds to find a pool of drug candidates with the desired properties (or “hits”). As a result of additional tests, the pool is further narrowed down, and only the most promising compounds (or “leads”) are selected.
In silico screening approaches are a valuable tool since they help speed up this stage and save resources. Yet, in vitro assays are for the time being still necessary to validate bioinformatic predictions.
Lead optimization
Following the identification of leads, a great amount of effort is devoted to significantly optimizing numerous properties of the leads, including their biological activity. As expected, this involves the iterative work of chemists who design, synthesize, and characterize new compounds. So as data is gathered after each round of tests, a better picture of how the chemical structure and activity are related is built up, fueling optimization.
Similar to what occurs during the Hit Discovery phase, in vitro pharmacology assays are used to generate high-quality data that provides precise information on the biological properties of the compounds tested.
The lead optimization phase culminates in the identification of one or more drug candidates to be assessed during the pre-clinical trials.
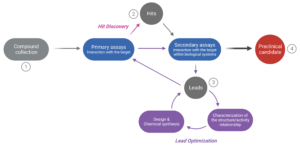
Figure 1. The drug discovery cycle. The path from a large collection of potentially efficacious compounds (1) to selecting one or more preclinical candidates (4) involves several steps. In the “Hit Discovery” phase, numerous molecules are screened to evaluate their interaction with the target (primary assays). Yet, only a small number (the “hits”) will be selected for further evaluation (2). Their interaction with the target in a biological context is assessed (secondary assays involving in vitro cell cultures or animal testing) to narrow down the pool of hits. The most promising compounds (the “leads”) are selected for optimization during the “Lead Optimization” phase (3). At this stage, the leads are characterized at the structure and activity level, which helps improve the design and chemical synthesis of new but structurally-related compounds. The new compounds are re-assessed through primary and secondary assays, despite their assumed functional similarity. This iterative process continues until a compound yielding satisfactory results is identified (selection of a “Preclinical candidate”).
In Vitro Pharmacology in Pre-Clinical Trials
On average, only 1 in 5,000 compounds that enter pre-clinical trials obtains approval for commercialization. The reason for this is that when a drug candidate enters pre-clinical trials, there is not yet enough information regarding the compound’s safety, toxicity, pharmacokinetics, and metabolism. Drug licensing authorities require the submission of a body of pre-clinical data on all of these parameters before testing any new drug on humans. Thus, they are assessed at the pre-clinical stage, typically through both in vitro and in vivo studies. The dose for a drug’s first use in clinical trials is also determined at this step.
At the pre-clinical trial stage, in vitro pharmacology assays can be used for many different purposes:
- profile compounds to guide pre-clinical in vivo safety and toxicity studies
- obtain high-quality data on the safety and toxicity of a drug candidate
- identify potential adverse effects early in the drug development process
- assess the potency and efficacy of a drug candidate against the target disease or condition
- gather data on pharmacokinetics and pharmacodynamics
- study the drug candidate’s mechanism of action in more depth
- evaluate the activity of biosimilar compounds
As we can observe, safety pharmacology is a crucial stage of the pre-clinical development process. Its purpose is to assess any potential undesirable effects of the drug on the body’s major systems. Although most safety testing was conducted on animals in the past, safety tests are currently making increasing use of in vitro cell and tissue models.
Can We Fully Replace Animal Testing?
The countless applications of in vitro pharmacology tests and assays lead to a fair question concerning drug development: Could in vitro pharmacology fully replace animal testing in the future?
Unfortunately, although it’s the case for the cosmetics and personal care industry, non-animal alternatives aren’t currently sufficient for safety and efficacy testing in the pharmaceutical industry. Due to extremely rigorous demands, animal testing is still used to investigate repeat-dose toxicity, carcinogenicity, and several other properties.
However, the ongoing pursuit of better animal welfare keeps driving the improvement and development of new in vitro technologies, so a future without animal testing is a realistic possibility.
Find an In Vitro Pharmacology Program to Suit Your Needs
At QIMA Life Sciences, we are committed to developing effective in vitro alternatives to animal experimentation methods. For this, we offer a comprehensive selection of services to support your research needs in biology and in vitro pharmacology.
Our scientists have extensive expertise and know-how that can be applied to a wide range of pre-clinical and clinical research projects. We offer customized technical solutions that can best meet your requirements, prerequisites, and objectives, with particular attention given to the feasibility study and personalized management of your projects. With our state-of-the-art facilities and over 20 years of experience supporting projects in the pharmaceutical and biotechnological industries, we are pleased to offer you dedicated project management support and consulting for your R&D process.
*
Ready to discuss a testing and research program that will suit the needs of your business?